

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

Chemistry, 22.06.2019 19:30
Anurse used a 0.02-mg/l solution of disinfection to clean a patients wound. what is the concentration of the solution expressed as a percentage?
Answers: 1

Chemistry, 23.06.2019 05:30
Elizabeth has two separate samples of the same substance. sample is in the liquid state, and the other is in the solid state. the two samples most likely differ in which property?
Answers: 1

Chemistry, 23.06.2019 06:30
What is the chemical formula for a compound between li and br? libr li2br libr2 libr3
Answers: 1
You know the right answer?
If the amount of energy required to break bonds in the reactants is more than the amount of energy r...
Questions

Physics, 27.02.2020 15:52



English, 27.02.2020 15:53



Mathematics, 27.02.2020 15:53

Biology, 27.02.2020 15:53

Mathematics, 27.02.2020 15:53




History, 27.02.2020 15:54

English, 27.02.2020 15:55


Social Studies, 27.02.2020 15:56


Mathematics, 27.02.2020 15:57

Chemistry, 27.02.2020 15:58

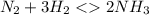
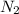 contains one N ≡ N triple bond (Bond breaking 946 KJ per mol)
contains one N ≡ N triple bond (Bond breaking 946 KJ per mol)
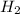 contains a single H-H bond (bond breaking 436KJ per mol)
contains a single H-H bond (bond breaking 436KJ per mol)
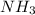 contains 3 N-H single bonds (389 KJ per mol)
contains 3 N-H single bonds (389 KJ per mol)


