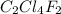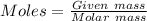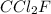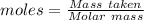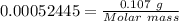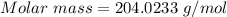Chemistry, 27.09.2019 02:30 tateandvioletAHS14AY
Analysis of a gaseous chlorofluorocarbon, cclxfy, shows that it contains 11.79% c and 69.57% cl. in another experiment, you find that 0.107 g of the compound fills a 458-ml flask at 25 °c with a pressure of 21.3 mm hg. what is the molecular formula of the compound?

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 20:30
In which layer of earth do most earthauakes occur a_ inner core b_outer core c_mantle d_crust
Answers: 1

Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3
You know the right answer?
Analysis of a gaseous chlorofluorocarbon, cclxfy, shows that it contains 11.79% c and 69.57% cl. in...
Questions

English, 14.07.2020 01:01



Mathematics, 14.07.2020 01:01

Mathematics, 14.07.2020 01:01




English, 14.07.2020 01:01

Mathematics, 14.07.2020 01:01

Mathematics, 14.07.2020 01:01





Mathematics, 14.07.2020 01:01


Mathematics, 14.07.2020 01:01

Mathematics, 14.07.2020 01:01