
Which of the following statements concerning the attraction of ions to polar molecules is/are correct? 1. the force of attraction between an ion and a polar molecule is inversely proportional to the square of the distance between the center of the ion and the oppositely charged pole of the dipole. 2. the higher the ion charge, the stronger the attraction between the ion and a polar molecule. 3. the greater the magnitude of the dipole, the greater the attraction between the ion and a polar molecule.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
What is the relationship of air masses and the temperature of oceans?
Answers: 1

Chemistry, 22.06.2019 04:00
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1

Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3

Chemistry, 22.06.2019 06:00
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2
You know the right answer?
Which of the following statements concerning the attraction of ions to polar molecules is/are correc...
Questions

Mathematics, 22.11.2020 05:50

Mathematics, 22.11.2020 05:50



Business, 22.11.2020 05:50

Mathematics, 22.11.2020 05:50

Mathematics, 22.11.2020 05:50

Mathematics, 22.11.2020 05:50

Mathematics, 22.11.2020 05:50

Mathematics, 22.11.2020 05:50

Physics, 22.11.2020 05:50

Business, 22.11.2020 05:50

Mathematics, 22.11.2020 05:50


Mathematics, 22.11.2020 05:50



Mathematics, 22.11.2020 05:50


Biology, 22.11.2020 05:50

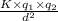
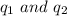 are the charges on the dipole and ion respectively.
are the charges on the dipole and ion respectively.

