Chemistry, 26.09.2019 21:30 dayanaraa61
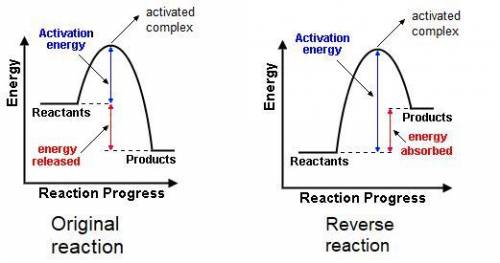
The gas phase reaction: cl(g) + hbr(g) →hcl(g) + br(g)has an overall enthalpy change of −66 kj. the activation energy for the reaction is 7 kj. a) draw the potential energy curve for the reaction and label ea, ∆e, and the activated complex or transition state. b) draw the potential curve for the reverse reaction. what is ea? what is ∆e?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Ahypothrticalax type of ceramic material is known to have a density of 2.10 g/cm3 and a unit cell of cubic symmetry with a cell edge length of 0.57 nm. the atomic weights of the a and x elements are 28.5and 30.0 g/mol, respectively. on the basis of this information, which of the following crystal structures is (are) possible for this material: sodium chloride, cesium chloride, or zinc blende
Answers: 1

Chemistry, 22.06.2019 07:00
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2

Chemistry, 22.06.2019 11:00
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2
You know the right answer?
The gas phase reaction: cl(g) + hbr(g) →hcl(g) + br(g)has an overall enthalpy change of −66 kj. the...
Questions

Mathematics, 26.10.2021 21:00

Physics, 26.10.2021 21:00



Mathematics, 26.10.2021 21:00

Biology, 26.10.2021 21:00





English, 26.10.2021 21:00



Mathematics, 26.10.2021 21:00


Chemistry, 26.10.2021 21:00

Social Studies, 26.10.2021 21:00

Mathematics, 26.10.2021 21:00

Mathematics, 26.10.2021 21:00

Computers and Technology, 26.10.2021 21:00