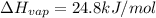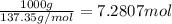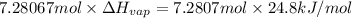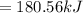Chemistry, 26.09.2019 19:30 Kekkdkskdkdk
Freon-11, ccl3f has been commonly used in air conditioners. it has a molar mass of 137.35 g/mol and its enthalpy of vaporization is 24.8 kj/mol at its normal boiling point of 24c. ideally how much energy in the form of heat is removed from a room by an air conditioner that evaporates 1.00 kg of freon-11?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Imagine that you’re getting ready to move to a new city. when people move, they are influenced by push factors and pull factors, and you have many reasons for your move. which of the following factors is an example of a pull factor? a. wanting to move because you’ve found a great new school somewhere new b. needing to move because there are not enough resources in your old hometown c. being forced to move because your old home is gone d. having to move because there are no jobs in your current hometown
Answers: 1

Chemistry, 21.06.2019 22:50
2. you__turn left on a red light if you are in the left-most lane of a one-way street, you're turning into the left-most lane of a one-way street, and no nearby sign prohibits the turn.
Answers: 2

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3
You know the right answer?
Freon-11, ccl3f has been commonly used in air conditioners. it has a molar mass of 137.35 g/mol and...
Questions



Mathematics, 11.11.2020 02:10


Social Studies, 11.11.2020 02:10

Mathematics, 11.11.2020 02:10



Biology, 11.11.2020 02:10




Mathematics, 11.11.2020 02:10

Mathematics, 11.11.2020 02:10



Physics, 11.11.2020 02:10

Chemistry, 11.11.2020 02:10


Mathematics, 11.11.2020 02:20