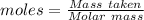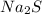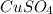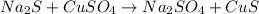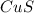What is the percent yield of cus for the following reaction given that you start with 15.5 g of na2s and 12.1 g cuso4? the actually amount of cus produced was 3.05 g. reaction: na2s + cuso4 → na2so4 + cus (a) 16.1% (b) 42.1% (c) 18.93% (d) 7.25% (e) not enough information

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:30
What metric units would you use to measure the thickness of a key
Answers: 3


Chemistry, 22.06.2019 17:10
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1

Chemistry, 23.06.2019 00:20
4. propanol and isopropanol are isomers. this means that they have a) the same molecular formula but different chemical properties. b) different molecular formulas but the same chemical properties. c) the same molecular formula and the same chemical properties. d) the same molecular formula but represent different states of the compound
Answers: 3
You know the right answer?
What is the percent yield of cus for the following reaction given that you start with 15.5 g of na2s...
Questions



Mathematics, 29.11.2021 20:50

History, 29.11.2021 20:50



Mathematics, 29.11.2021 20:50


Mathematics, 29.11.2021 20:50

Physics, 29.11.2021 20:50


Mathematics, 29.11.2021 20:50




Mathematics, 29.11.2021 20:50

Arts, 29.11.2021 20:50