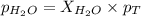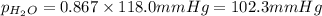Chemistry, 26.09.2019 19:00 Tyrant4life
What is the equilibrium partial pressure of water vapor above a mixture of 62.9 g h2o and 33.2 g hoch2ch2oh at 55 °c. the partial pressure of pure water at 55.0 °c is 118.0 mm hg. assume ideal behavior for the solution.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Achef makes salad dressing by mixing oil, vinegar, and spices, as shown. which type of matter is the salad dressing?
Answers: 1

Chemistry, 22.06.2019 00:10
Select the correct answer. which phrase correctly describes temperature? o a. average rotational kinetic energy of the particles in an object o b. average energy of the particles in an object c. average translational kinetic energy of the particles in an object od. all energy possessed by the particles in an object
Answers: 1

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

Chemistry, 22.06.2019 21:00
Read "who built the pyramids? ”. leave this link open while you answer the questions throughout the assignment. give at least two reasons why some people claim the pyramids of giza were constructed by aliens.
Answers: 1
You know the right answer?
What is the equilibrium partial pressure of water vapor above a mixture of 62.9 g h2o and 33.2 g hoc...
Questions

Mathematics, 16.09.2021 05:20

Medicine, 16.09.2021 05:20

English, 16.09.2021 05:20








Mathematics, 16.09.2021 05:20




Arts, 16.09.2021 05:20

Chemistry, 16.09.2021 05:20


Mathematics, 16.09.2021 05:20


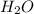 is 102.3 mmHg.
is 102.3 mmHg.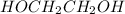 = 33.2 g
= 33.2 g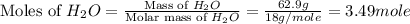
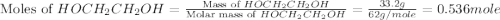
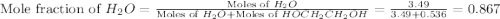
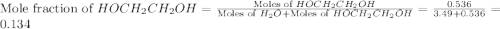
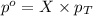
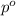 = partial pressure of water vapor
= partial pressure of water vapor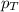 = total pressure of gas
= total pressure of gas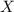 = mole fraction of water vapor
= mole fraction of water vapor