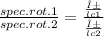An aqueous solution containing 10 g of an optically pure compound was diluted to 500 ml with water and was found to have a specific rotation of −129°. if this solution were mixed with 500 ml of a solution containing 7 g of a racemic mixture of the compound, what would the specific rotation of the resulting mixture of the compound? what would be its optical purity?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1

Chemistry, 22.06.2019 16:30
For the reaction shown, calculate how many moles of no2 form when each of the following completely reacts. 2n2o5(g)→4no2(g)+o2(g) part a 1.0 mol n2o5 express your answer using two significant figures. nothing mol m o l request answer part b 5.4 mol n2o5 express your answer using two significant figures.
Answers: 2

Chemistry, 22.06.2019 19:00
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1

Chemistry, 23.06.2019 10:30
If a 20.0ml test tube measures 15.0cm, what is the length in meters?
Answers: 1
You know the right answer?
An aqueous solution containing 10 g of an optically pure compound was diluted to 500 ml with water a...
Questions


Mathematics, 22.09.2019 21:30



Mathematics, 22.09.2019 21:30


Chemistry, 22.09.2019 21:30


Biology, 22.09.2019 21:30

Business, 22.09.2019 21:30

Biology, 22.09.2019 21:30


Mathematics, 22.09.2019 21:30

English, 22.09.2019 21:30

Physics, 22.09.2019 21:30

Mathematics, 22.09.2019 21:30


History, 22.09.2019 21:30

English, 22.09.2019 21:30