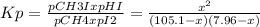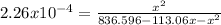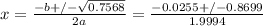Chemistry, 26.09.2019 17:30 Demondevilg
Methane, ch4, reacts with i2 according to the reaction ch4(g)+i2(g)⇌ch3i(g)+hi(g)
at 630 k, kp for this reaction is 2.26×10−4. a reaction was set up at 630 k with initial partial pressures of methane of 105.1 torr and of 7.96 torr for i2.
calculate the pressure, in torr, of ch4.
express your answer to four significant figures and include the appropriate units.
calculate the pressure, in torr, of i2.
express your answer to three significant figures and include the appropriate units.
calculate the pressure, in torr, of ch3i.
express your answer to three significant figures and include the appropriate units.
calculate the pressure, in torr, of hi.
express your answer to three significant figures and include the appropriate units.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Chemistry, 22.06.2019 21:30
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2

You know the right answer?
Methane, ch4, reacts with i2 according to the reaction ch4(g)+i2(g)⇌ch3i(g)+hi(g)
at 630 k, kp...
at 630 k, kp...
Questions

English, 20.10.2020 18:01


Health, 20.10.2020 18:01






History, 20.10.2020 18:01

Geography, 20.10.2020 18:01

Chemistry, 20.10.2020 18:01

Mathematics, 20.10.2020 18:01

Mathematics, 20.10.2020 18:01

Mathematics, 20.10.2020 18:01





Mathematics, 20.10.2020 18:01

Mathematics, 20.10.2020 18:01

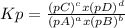 , where p is the partial pressure in the equilibrium. By the reaction given:
, where p is the partial pressure in the equilibrium. By the reaction given: