
Chemistry, 26.09.2019 18:00 zoeatlowapple
Consider the reaction between sodium metal and fluorine gas to form sodium fluoride. using oxidation states, how many electrons would each sodium atom lose, and how many electrons would each fluorine atom gain? #e- each sodium atom would loose? #e- each fluorine atom would gain? how many sodium atoms are needed to react with one fluorine molecule?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Five students had to answer the question how are elements arranged in a periodic tabledamon: i think the elements are arranged by increasing massflo: i think the elements are arranged according to their properties sienna: i think the elements are arranged by when their discovers kyle: i think the elements are arranged according to how common they areglenda: i don't agree with any of themwho is right
Answers: 1

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3

Chemistry, 22.06.2019 12:40
Consider the directing effects of the substituents on salicylamide and predict the possible structures of the iodination products. which do you think will be the major product?
Answers: 1

Chemistry, 22.06.2019 14:00
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3
You know the right answer?
Consider the reaction between sodium metal and fluorine gas to form sodium fluoride. using oxidation...
Questions





History, 16.01.2020 20:31

Health, 16.01.2020 20:31










Social Studies, 16.01.2020 20:31




![[Ne]3s^1](/tpl/images/0265/3273/4a392.png) .
.
![[He]2s^22p^5](/tpl/images/0265/3273/da8fe.png) .
.
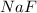 .
.

