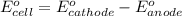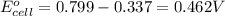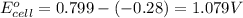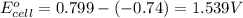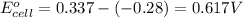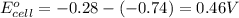Chemistry, 26.09.2019 16:30 savage5447
The standard reduction potentials of the following half-reactions are given in appendix e in the textbook:
ag+(aq)+e−→ag(s)= .799
cu2+(aq)+2e−→cu(s)= .337
ni2+(aq)+2e−→ni(s)= -.28
cr3+(aq)+3e−→cr(s). = -.74
1. determine which combination of these half-cell reactions leads to the cell reaction with the largest positive cell emf.
1st and 2nd,
1st and 3rd,
1st and 4th,
2nd and 3rd,
3rd and 4th.
it isn't the first or last one because i have gotten it wrong twice.
2. calculate the value of this emf.
3. then determine which combination is the smallest and calculate the emf.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
What is driving behind plate tectonics (plate movment)? a) gravity only b) inertia c) convection and gravity d) the sun theres no option for science so i picked chemistry. plz
Answers: 2

Chemistry, 22.06.2019 09:00
Ineed to find the answer of this question because i dont understand it
Answers: 1

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 22.06.2019 21:00
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3
You know the right answer?
The standard reduction potentials of the following half-reactions are given in appendix e in the tex...
Questions



Mathematics, 14.03.2020 05:06


Biology, 14.03.2020 05:06



Mathematics, 14.03.2020 05:06












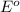 potential will always get reduced and will undergo reduction reaction.
potential will always get reduced and will undergo reduction reaction.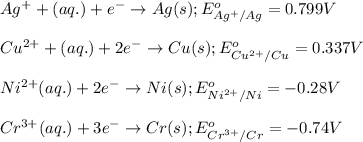
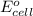 of the reaction, we use the equation:
of the reaction, we use the equation: