
Chemistry, 25.09.2019 02:01 hlgerardip4wbhx
Determine the concentrations of mgcl2, mg2+, and cl− in a solution prepared by dissolving 2.52 × 10−4 g mgcl2 in 2.00 l of water. express all three concentrations in molarity. additionally, express the concentrations of the ionic species in parts per million (ppm).

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:40
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na,so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1



Chemistry, 22.06.2019 17:10
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2
You know the right answer?
Determine the concentrations of mgcl2, mg2+, and cl− in a solution prepared by dissolving 2.52 × 10−...
Questions



Mathematics, 20.06.2021 05:30

History, 20.06.2021 05:30







History, 20.06.2021 05:30


Mathematics, 20.06.2021 05:40


English, 20.06.2021 05:40

Mathematics, 20.06.2021 05:40


Mathematics, 20.06.2021 05:40


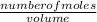
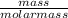
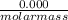 = 0.000252moles
= 0.000252moles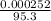 = 2.64 x 10⁻⁶moldm⁻³
= 2.64 x 10⁻⁶moldm⁻³

