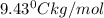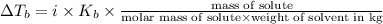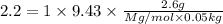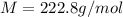A2.60 gram sample of a compound know to contain only indium and chlorine is dissolved in 50.0 g of tin(iv) chloride (kb = 9.43oc kg mol-1). the normal boiling point is raised from 114.1oc for pure sncl4 to 116.3oc for the solution. what is the molecular weight and probable molecular formula of the solute?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2

Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2

Chemistry, 23.06.2019 01:30
Which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1

Chemistry, 23.06.2019 02:20
In a chemical reaction, the final amount of the products is determined by the a. universal gas law b. law of definite proportions c. air pressure d. temperature e. none of the above me
Answers: 2
You know the right answer?
A2.60 gram sample of a compound know to contain only indium and chlorine is dissolved in 50.0 g of t...
Questions

History, 24.08.2019 16:30



Mathematics, 24.08.2019 16:30

Mathematics, 24.08.2019 16:30



Biology, 24.08.2019 16:30


Biology, 24.08.2019 16:30

Mathematics, 24.08.2019 16:30


Mathematics, 24.08.2019 16:30


History, 24.08.2019 16:30

Chemistry, 24.08.2019 16:30

Chemistry, 24.08.2019 16:30


Social Studies, 24.08.2019 16:30

Health, 24.08.2019 16:30



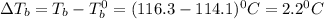 = elevation in boiling point
= elevation in boiling point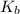 = boiling point constant =
= boiling point constant =