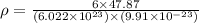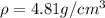Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3


Chemistry, 22.06.2019 18:00
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2

Chemistry, 22.06.2019 23:40
What energy conversion occurs when a sling shot is used to shoot a rock across the room? (2 points) question 2 options: 1) stored mechanical energy is converted to mechanical energy. 2) stored mechanical energy is converted to radiant energy. 3) gravitational energy is converted to radiant energy. 4) gravitational energy is converted to mechanical energy.
Answers: 1
You know the right answer?
Titanium has an hcp unit cell for which the ratio of the lattice parameters cais 1.58. if the radius...
Questions





Physics, 14.08.2020 20:01




Mathematics, 14.08.2020 20:01






Mathematics, 14.08.2020 20:01

Mathematics, 14.08.2020 20:01

Spanish, 14.08.2020 20:01



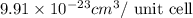
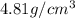
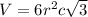
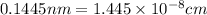
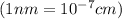
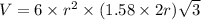
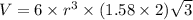
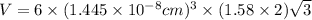
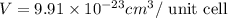
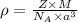
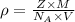 ..........(1)
..........(1)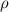 = density of Ti = ?
= density of Ti = ?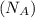 = Avogadro's number =
= Avogadro's number = 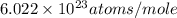
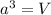 = volume of unit cell =
= volume of unit cell =