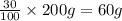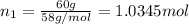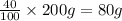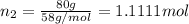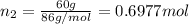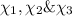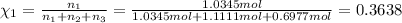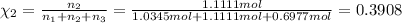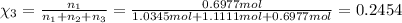Chemistry, 25.09.2019 02:00 jaylabazemore
Two hundred kg of liquid contains 30% butane, 40% pentane, and the rest hexane (mass %) determine: the mole fraction composition of the liquid the mass fraction composition on hexane free basis 1. 2.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no]2[o2]
Answers: 3

Chemistry, 22.06.2019 06:00
What type of electromagnetic radiation has a shorter wavelength than blue light
Answers: 2


Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2
You know the right answer?
Two hundred kg of liquid contains 30% butane, 40% pentane, and the rest hexane (mass %) determine:...
Questions



Mathematics, 24.01.2021 14:00


Chemistry, 24.01.2021 14:00



Mathematics, 24.01.2021 14:00

Chemistry, 24.01.2021 14:00


Mathematics, 24.01.2021 14:00

Chemistry, 24.01.2021 14:00

Mathematics, 24.01.2021 14:00




Chemistry, 24.01.2021 14:00

Mathematics, 24.01.2021 14:00