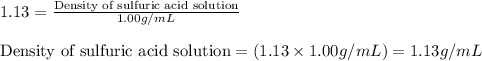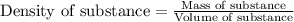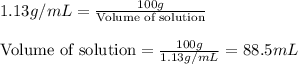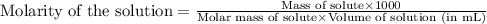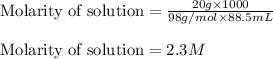Chemistry, 25.09.2019 02:00 madelyngv97
Find the molar concentration of sulfuric acid in a 20.0 wt% solution of sulfuric acid in water (sg=1.13).

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2

Chemistry, 23.06.2019 00:50
What is the enthalpy of combustion (per mole) of c4h10 (g)? –2,657.5 kj/mol –5315.0 kj/mol –509.7 kj/mol –254.8 kj/mol
Answers: 1

Chemistry, 23.06.2019 01:00
Na chemical reaction, activation energy increases the of the reactants. this outcome causes the particles to collide, which results in the of new products.
Answers: 2

Chemistry, 23.06.2019 02:40
Calculate the standard enthalpy of formation of liquid methanol, ch3oh(l), using the following information: c(graphite) + o2 latex: \longrightarrow ⟶ co2(g) latex: \delta δ h° = –393.5 kj/mol h2(g) + o2 latex: \longrightarrow ⟶ h2o(l) latex: \delta δ h° = –285.8 kj/mol ch3oh(l) + o2(g) latex: \longrightarrow ⟶ co2(g) + 2h2o(l) latex: \delta δ h° = –726.4 kj/mol
Answers: 3
You know the right answer?
Find the molar concentration of sulfuric acid in a 20.0 wt% solution of sulfuric acid in water (sg=1...
Questions

Advanced Placement (AP), 03.12.2021 06:50

Mathematics, 03.12.2021 06:50



Mathematics, 03.12.2021 06:50


Mathematics, 03.12.2021 06:50

English, 03.12.2021 06:50

Mathematics, 03.12.2021 06:50


Mathematics, 03.12.2021 06:50




Mathematics, 03.12.2021 06:50

Mathematics, 03.12.2021 06:50

Social Studies, 03.12.2021 06:50