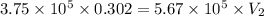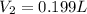Chemistry, 25.09.2019 01:20 JimmySample7
Acompressed cylinder of gas contains 45.6 mol of n2 gas at a pressure of 3.75 x 105 pa and a temperature of 23.6°c. what volume of gas has been released into the atmosphere if the final pressure in the cylinder is 5.67 x 105 pa? assume ideal behavior and that the gas temperature is unchanged.

Answers: 3


Another question on Chemistry

Chemistry, 20.06.2019 18:02
All living organisms are composed of a. at least three cells. b. one or more cells. c. only one cell. d. at least 100 cells.
Answers: 2

Chemistry, 21.06.2019 20:30
This chart represents the melting point of several substance. what besy explains the high melting point of the salt?
Answers: 2

Chemistry, 22.06.2019 00:30
This active feature of earth's crust in building mountain ranges as well as islands. this feature is a a) cavern. b) earthquake. c) mountain. d) volcano.
Answers: 2

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1
You know the right answer?
Acompressed cylinder of gas contains 45.6 mol of n2 gas at a pressure of 3.75 x 105 pa and a tempera...
Questions

Mathematics, 01.08.2019 03:10






Social Studies, 01.08.2019 03:10

Mathematics, 01.08.2019 03:10

Computers and Technology, 01.08.2019 03:10











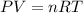
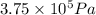 = 3675 atm (1 kPa= 0.0098 atm)
= 3675 atm (1 kPa= 0.0098 atm)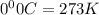
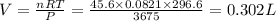
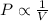 (At constant temperature and number of moles)
(At constant temperature and number of moles)
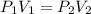
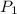 = initial pressure of gas =
= initial pressure of gas = 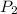 = final pressure of gas =
= final pressure of gas = 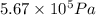
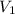 = initial volume of gas = 0.302 L
= initial volume of gas = 0.302 L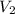 = final volume of gas = ?
= final volume of gas = ?