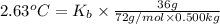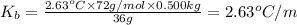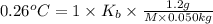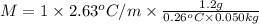The benzene boiling temperature (c6h6) is 80.1ºc dissolving 36 g pentane, c5h12 at 500 g benzene increases the boiling point of the solution to 82.73ºc
a. consider the benzene boiling point constant. show calculations.
b. in dissolving 1.2 g of unknown solute in 50 g of benzene, a solution with a boiling point of 80.36ºc is obtained, which is the molar mass of the solute (assume that i = 1) (show calculations)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2

Chemistry, 22.06.2019 20:10
Insoluble sulfide compounds are generally black in color. which of the following combinations could yield a black precipitate? check all that apply. na2s(aq)+kcl(aq) li2s(aq)+pb(no3)2(aq) pb(clo3)2(aq)+nano3(aq) agno3(aq)+kcl(aq) k2s(aq)+sn(no3)4(aq)
Answers: 1

You know the right answer?
The benzene boiling temperature (c6h6) is 80.1ºc dissolving 36 g pentane, c5h12 at 500 g benzene inc...
Questions







Chemistry, 04.02.2020 22:55


Social Studies, 04.02.2020 22:55

Biology, 04.02.2020 22:55



History, 04.02.2020 22:55




Mathematics, 04.02.2020 22:55



Mathematics, 04.02.2020 22:55

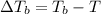
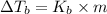
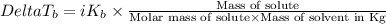
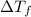 =Elevation in boiling point
=Elevation in boiling point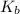 = boiling point constant od solvent= 3.63 °C/m
= boiling point constant od solvent= 3.63 °C/m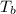 =82.73°C
=82.73°C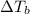 = 82.73°C - 80.1°C = 2.63°C
= 82.73°C - 80.1°C = 2.63°C