
Agas mixture of 20 moles nitrogen and 70 moles hydrogen is fed to a reactor in which ammonia is produced. assuming that the reaction proceeds to completion, determine the composition of the exit gas stream both in mole and mass %. if 75% of nitrogen gets converted, what will be the corresponding fractions?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1

Chemistry, 22.06.2019 16:50
Which element is least likely to undergo a chemical reaction
Answers: 3

Chemistry, 22.06.2019 19:30
Describe the forces both attractive and repulsive that occur as two atoms move closer together.
Answers: 1

Chemistry, 23.06.2019 00:30
You are attempting to recrystallize a crude product mixture. you add the appropriate amount of hot solvent and are allowing the solution to slowly cool to room temperature. however, at room temperature no crystals have appeared, which of the following methods should be used to induce crystallization? choose all correct answers. a) place the flask in an ice bath. b) swirl the contents of the flask. c) add a small seed crystal of the desired product. d) scratch the inside of the glassware using a stir rod. it can be multiple choices
Answers: 3
You know the right answer?
Agas mixture of 20 moles nitrogen and 70 moles hydrogen is fed to a reactor in which ammonia is prod...
Questions



Biology, 15.06.2021 20:50



History, 15.06.2021 20:50

History, 15.06.2021 20:50


Mathematics, 15.06.2021 20:50





Mathematics, 15.06.2021 20:50


Mathematics, 15.06.2021 20:50

Mathematics, 15.06.2021 20:50


Physics, 15.06.2021 20:50

Mathematics, 15.06.2021 20:50

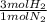 = 60 moles of H₂(g)
= 60 moles of H₂(g) 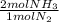 = 40 moles of NH₃(g)
= 40 moles of NH₃(g)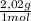 = 20,2 g of H₂
= 20,2 g of H₂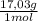 = 681,2 g of NH₃
= 681,2 g of NH₃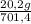 ₓ 100 = 2,9% H₂;
ₓ 100 = 2,9% H₂; 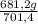 ₓ100 = 97,1% NH₃
ₓ100 = 97,1% NH₃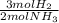 = 45 moles of H₂(g)
= 45 moles of H₂(g) 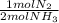 = 15 moles of N₂(g)
= 15 moles of N₂(g) 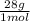 = 140 g of N₂
= 140 g of N₂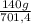 ₓ 100 =20% N₂;
ₓ 100 =20% N₂; 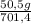 ₓ 100 = 7,2% H₂;
ₓ 100 = 7,2% H₂; 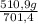 ₓ100 = 72,8% NH₃
ₓ100 = 72,8% NH₃

