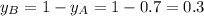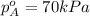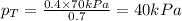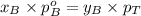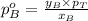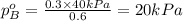Components a and b form ideal solution. at 350 k, a liquid mixture containing 40% (mole) a is in equilibrium with a vapour containing 70% (mole) a. if the vapour pressure of a at 350 k is 70 kpa, what is the vapour pressure of b? (b) 20 kpa (d) 12 kpa (а) 25 kpa (c) 40 kpa

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Which answer lists the fundamental forces in order from strongest to weakest
Answers: 1

Chemistry, 21.06.2019 22:30
Naoki's bicycle has a mass of 10 kg. if naoki sits on her bicycle and starts pedaling with a force of 168 n, causing an acceleration of 2.8 m/s2, what is naoki's mass?
Answers: 1

Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3
You know the right answer?
Components a and b form ideal solution. at 350 k, a liquid mixture containing 40% (mole) a is in equ...
Questions

Mathematics, 06.11.2019 19:31





History, 06.11.2019 19:31

Mathematics, 06.11.2019 19:31





Mathematics, 06.11.2019 19:31

Chemistry, 06.11.2019 19:31



Health, 06.11.2019 19:31




Mathematics, 06.11.2019 19:31

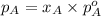 .............(1)
.............(1)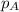 = partial vapor pressure of A
= partial vapor pressure of A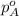 = vapor pressure of pure substance A
= vapor pressure of pure substance A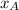 = mole fraction of A
= mole fraction of A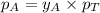 .............(2)
.............(2)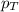 = total pressure of the mixture
= total pressure of the mixture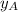 = mole fraction of A
= mole fraction of A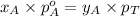
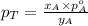 ............(3)
............(3)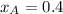 and
and 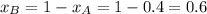
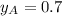 and
and