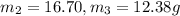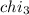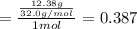Chemistry, 25.09.2019 00:20 latdoz0952
Aliquid mixture contains water (h2o, mw = 18.0), ethanol (c2h5oh, mw = 46.0) and methanol (ch3oh, mw = 32.0). using two different analytical techniques to analyze the mixture, it was determined that the water mole fraction was 0.250 while the water mass fraction was 0.134. determine the mole fraction ethanol (c2h5oh) and the mole fraction methanol (ch3oh) in the solution. report the values to the correct number of significant figures.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 01:00
Which of the following is always a reactant in a combustion reaction? oxygen nitrogen hydrogen carbon
Answers: 1

Chemistry, 22.06.2019 03:00
Compare the valence electron configuration of the nobles gas elements seen here. what statement is correct?
Answers: 2

Chemistry, 22.06.2019 04:30
What are the three major branches of natural science? • earth and space science, life science, physical science •earth and space science, physical science, chemistry •physical science, life science, chemistry •life science, chemistry, physics
Answers: 1
You know the right answer?
Aliquid mixture contains water (h2o, mw = 18.0), ethanol (c2h5oh, mw = 46.0) and methanol (ch3oh, mw...
Questions

Mathematics, 27.01.2020 08:31

Computers and Technology, 27.01.2020 08:31


Mathematics, 27.01.2020 08:31





Mathematics, 27.01.2020 08:31


Chemistry, 27.01.2020 08:31


Mathematics, 27.01.2020 08:31

Mathematics, 27.01.2020 08:31






Mathematics, 27.01.2020 08:31

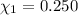
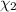
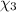
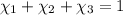
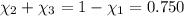
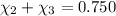
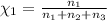
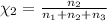
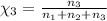
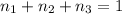
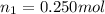



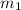
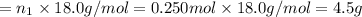

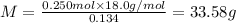


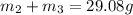 ..[1]
..[1]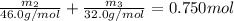
 ..[2]
..[2]