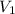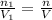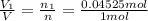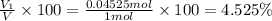Chemistry, 24.09.2019 23:30 zanaplen27
Deep sea divers use a mixture of helium and oxygen to breathe. assume that a diver is going to a depth of 150 feet where the total pressure is 4.42 atm. the partial pressure of oxygen at this depth is to be maintained at 0.20 atm, the same as at sea level. what must be the percent by volume of oxygen in the gas mixture?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1

Chemistry, 22.06.2019 13:00
Adepositional also feature that forms where a stream enters a lake or an ocean is a
Answers: 2

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1
You know the right answer?
Deep sea divers use a mixture of helium and oxygen to breathe. assume that a diver is going to a dep...
Questions

English, 21.04.2021 01:00


Chemistry, 21.04.2021 01:00

History, 21.04.2021 01:00

Mathematics, 21.04.2021 01:00

Mathematics, 21.04.2021 01:00



Social Studies, 21.04.2021 01:00



English, 21.04.2021 01:00

Mathematics, 21.04.2021 01:00





Mathematics, 21.04.2021 01:00

Mathematics, 21.04.2021 01:00

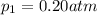
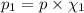 (Dalton law of partial pressure)
(Dalton law of partial pressure)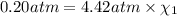
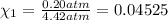
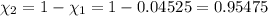
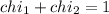
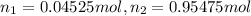
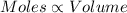 (At temperature and pressure)
(At temperature and pressure)