
Chemistry, 24.09.2019 23:11 celibe9391
Aluminum chloride, aich is an inexpensive reagent used in many industrial processes. it is made by treating scrap aluminum with chlorine according to the following equation. 2 aln + 3 cla) → 2 alcl3() a) if 13,49 g of al and 35.45 g of cl2 are allowed to react, how much alcl is produced? b) how many grams of the excess reactant is left? !

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1


Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2

You know the right answer?
Aluminum chloride, aich is an inexpensive reagent used in many industrial processes. it is made by t...
Questions


Mathematics, 26.05.2021 05:50




Mathematics, 26.05.2021 05:50

Mathematics, 26.05.2021 05:50

Chemistry, 26.05.2021 05:50

Mathematics, 26.05.2021 06:00

Mathematics, 26.05.2021 06:00



Biology, 26.05.2021 06:00


English, 26.05.2021 06:00





Mathematics, 26.05.2021 06:00

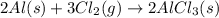
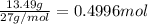
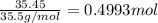
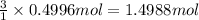 of chlorine gas
of chlorine gas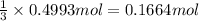 of aluminum
of aluminum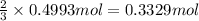 of aluminium chloride .
of aluminium chloride .

