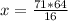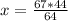The reaction is as follows: ch4 + 202 + co2 + 2h2o if we have 71 kg/hr of ch4 reacting with 67 kg/hr of 02, at what rate co2 will be generated in kg/hr? molecular weight: c-12 kg/kmol h-1 kg/kmol 0 - 16 kg/kmol select one: o a. 46.062 o b. 195.250 o o o c. 1.047 d. 92.125 e. 24.364

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
14. many depressants reduce small muscle control, making it harder for a. you to steer b. your mind to consider complex problems c. the eye to scan, focus, or stay still d. the kidneys to filter alcohol out of the bloodstream
Answers: 3

Chemistry, 22.06.2019 04:30
In which phase(s) do the molecules take the shape of the container?
Answers: 1

Chemistry, 22.06.2019 14:30
Which of the following is not one of the steps in the scientific method a. hypothesize b. summarize c. analyze d. familiarize
Answers: 3

Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1
You know the right answer?
The reaction is as follows: ch4 + 202 + co2 + 2h2o if we have 71 kg/hr of ch4 reacting with 67 kg/h...
Questions

English, 08.02.2021 20:10




Mathematics, 08.02.2021 20:10


Mathematics, 08.02.2021 20:10






Engineering, 08.02.2021 20:10

Social Studies, 08.02.2021 20:10



Computers and Technology, 08.02.2021 20:10


Mathematics, 08.02.2021 20:10

Mathematics, 08.02.2021 20:10