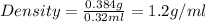Chemistry, 24.09.2019 20:00 prettygirllniyiaa
The active ingredient in aspirin is acetylsalicylic acid. in a lab class, a student uses paper chromatography to isolate another common ingredient of headache remedies. the sample of this ingredient had a mass of 384 mg and a volume of 0.32 cm3. looking at the following data, what was the other ingredient in the headache remedy? white table sugar caffeine sodium chloride d -0.70 g/ml d 1.2 g/ml d 2.2 g/ml

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 19:00
Structure of the atoms: discovery of the nucleus in 1909i need answering all of these questions
Answers: 3

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3

Chemistry, 23.06.2019 05:30
Based on the formulas, select the compounds below that are covalent: kbr sif4 al2o3 co2 naco3 s7o2 pcl3 fe3n2 h2o s2f10
Answers: 3

Chemistry, 23.06.2019 12:10
Which structure is a valid representation of a hydrocarbon molecule?
Answers: 2
You know the right answer?
The active ingredient in aspirin is acetylsalicylic acid. in a lab class, a student uses paper chrom...
Questions

Social Studies, 09.11.2020 20:20

English, 09.11.2020 20:20

Mathematics, 09.11.2020 20:20






Mathematics, 09.11.2020 20:20

Mathematics, 09.11.2020 20:20


History, 09.11.2020 20:20



Mathematics, 09.11.2020 20:20


History, 09.11.2020 20:20

History, 09.11.2020 20:20


Mathematics, 09.11.2020 20:20

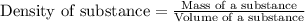
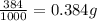 (1g=1000mg)
(1g=1000mg)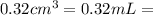 (Conversion factor:
(Conversion factor: 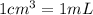 )
)