
Chemistry, 24.09.2019 20:00 leelee8335
You notice that the water in your friend's swimming pool is cloudy and that the pool walls are discolored at the water line. a quick analysis reveals that the ph of the water is 8.00 when it should be 7.20. the pool is 9.00 m wide, 15.0 m long, and has an average depth of 2.50m. what is the minimum (in the absence of any buffering capacity) volume (ml) of 12.0 wt% h2so4 (sg=1.080) that should be added to return the pool to the desired ph? in ml

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
Why is the structure of molecule important to its function?
Answers: 1

Chemistry, 22.06.2019 12:00
Ineed this asap part i: scientific method what is the difference between science and pseudoscience? what is the scientific method?
Answers: 2

Chemistry, 23.06.2019 00:30
What would be the original temperature of a gas that has a volume of 2.0 l and a pressure of 2.0 atm and an unknown temperature that the volume increased to 3.5 l in its pressure decreased to 1.0 atm if the final temperature is measured to be 11°c
Answers: 1

Chemistry, 23.06.2019 09:00
Which factor is likely to impact the possible number of compounds? presence of unlimited number of elements in the periodic table the inability of atoms to align perfectly with other atoms the ability of all elements to react with every other element all elements being equally reactive
Answers: 2
You know the right answer?
You notice that the water in your friend's swimming pool is cloudy and that the pool walls are disco...
Questions



Mathematics, 20.05.2021 21:10


Mathematics, 20.05.2021 21:10


English, 20.05.2021 21:10


Mathematics, 20.05.2021 21:10

Mathematics, 20.05.2021 21:10

Advanced Placement (AP), 20.05.2021 21:10


Mathematics, 20.05.2021 21:10


Mathematics, 20.05.2021 21:10



Biology, 20.05.2021 21:10


Mathematics, 20.05.2021 21:10

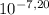 , thus,
, thus, 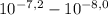 = 5,31x10⁻⁸ M
= 5,31x10⁻⁸ M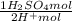 = 8,96x10⁻³ moles of H₂SO₄
= 8,96x10⁻³ moles of H₂SO₄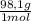 ×
× 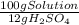 ×
× 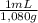 =
= 

