
Chemistry, 24.09.2019 01:20 RickandMorty420710
Agas of potassium chlorate molecules kclo3 all decompose into potassium chloride, kcl, and diatomic oxygen, o2. the products and reactants are in a closed container and can all be treated as ideal gases. a. fill in the smallest possible integers that allows the stoichiometry of the reaction equation to be correct: __ kclo3 → kcl o2b. if there are n molecules of potassium chlorate in the initial state, how many product molecules are there

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1


Chemistry, 22.06.2019 13:00
Imagine that you push on a large rock. at what point does your effort change the rock’s mechanical energy?
Answers: 1

Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1
You know the right answer?
Agas of potassium chlorate molecules kclo3 all decompose into potassium chloride, kcl, and diatomic...
Questions



History, 29.01.2021 21:20


History, 29.01.2021 21:20


Mathematics, 29.01.2021 21:20



Biology, 29.01.2021 21:20

Mathematics, 29.01.2021 21:20

Arts, 29.01.2021 21:20



Social Studies, 29.01.2021 21:20

Mathematics, 29.01.2021 21:20

Mathematics, 29.01.2021 21:20


Mathematics, 29.01.2021 21:20

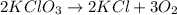
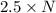 molecules of product.
molecules of product.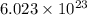 of particles.
of particles.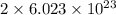 molecules of reactant give
molecules of reactant give 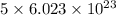 molecules of product
molecules of product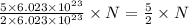 molecules of product.
molecules of product.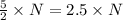 molecules of product are there.
molecules of product are there.

