Chemistry, 23.09.2019 21:10 sparky1234
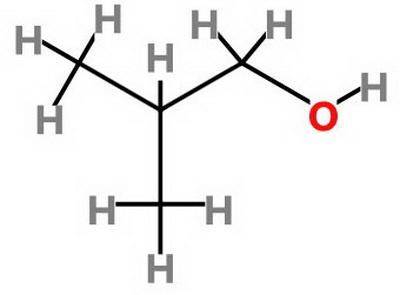
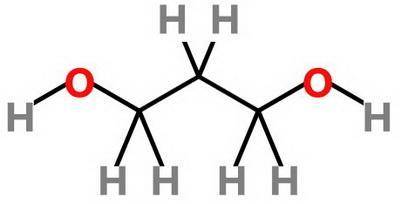
Which statement about 2‑methyl‑1‑propanol, (ch3)2chch2oh , and 1,3‑propanediol, hoch2ch2ch2oh is true? 2‑methyl‑1‑propanol is more soluble in water than 1,3‑propanediol because 2‑methyl‑1‑propanol has a smaller molecular mass. 2‑methyl‑1‑propanol is more soluble in water than 1,3‑propanediol because 2‑methyl‑1‑propanol forms fewer hydrogen bonds with water. 1,3‑propanediol is more soluble in water than 2‑methyl‑1‑propanol because 1,3‑propanediol has a smaller molecular mass. 1,3‑propanediol is more soluble in water than 2‑methyl‑1‑propanol because 1,3‑propanediol can form multiple hydrogen bonds with water.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:30
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 22.06.2019 14:30
Chemistry worksheet - i am not sure what they are asking for exactly?
Answers: 1

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1

Chemistry, 22.06.2019 23:00
Condensation happens when water vapor cools. true or false? ?
Answers: 2
You know the right answer?
Which statement about 2‑methyl‑1‑propanol, (ch3)2chch2oh , and 1,3‑propanediol, hoch2ch2ch2oh is tru...
Questions

Social Studies, 10.11.2020 23:10



Health, 10.11.2020 23:10

Mathematics, 10.11.2020 23:10


Mathematics, 10.11.2020 23:10

Biology, 10.11.2020 23:10

Mathematics, 10.11.2020 23:10


Mathematics, 10.11.2020 23:10


Mathematics, 10.11.2020 23:10

Mathematics, 10.11.2020 23:10


History, 10.11.2020 23:10




Physics, 10.11.2020 23:10