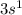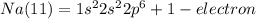Chemistry, 23.09.2019 19:00 NikkiZoeller
We all learned in our first course in chemistry that the most basic forms of matter are the elements, and that the smallest unit of an element is the atom. elements display properties unique to themselves, including the number of electrons located in what are referred to as atomic orbitals. these atomic orbitals are designated by type and energy as 1s, 2s, 2p, 3s, 3p, 4s, 3d, etc. q1. write out the electron configuration for sodium (na, element number 11). in what atomic orbital does the electron with the most energy reside?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 09:00
Identify the electromagnets with poles that are reversed from the electromagnet shown above
Answers: 3

Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1
You know the right answer?
We all learned in our first course in chemistry that the most basic forms of matter are the elements...
Questions





Health, 23.03.2021 22:10

Mathematics, 23.03.2021 22:10




Biology, 23.03.2021 22:10



Mathematics, 23.03.2021 22:10



Mathematics, 23.03.2021 22:10

Mathematics, 23.03.2021 22:10

Computers and Technology, 23.03.2021 22:10

English, 23.03.2021 22:10