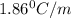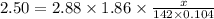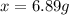Chemistry, 23.09.2019 18:10 pakabigail7116
What mass of na2so4 must be dissolved in 104 grams of water to lower the freezing point by 2.50 °c? the freezing point depression constant, kfp, of water is –1.86 °c/m. assume the van't hoff factor for na2so4 is 2.88.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 1

Chemistry, 22.06.2019 05:40
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

Chemistry, 22.06.2019 17:10
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1

Chemistry, 22.06.2019 23:30
What are the similarities between compounds and mixtures?
Answers: 3
You know the right answer?
What mass of na2so4 must be dissolved in 104 grams of water to lower the freezing point by 2.50 °c?...
Questions

English, 15.07.2019 04:30





Business, 15.07.2019 04:30

Social Studies, 15.07.2019 04:30


Mathematics, 15.07.2019 04:30

English, 15.07.2019 04:30


Mathematics, 15.07.2019 04:30

Mathematics, 15.07.2019 04:30

Mathematics, 15.07.2019 04:30




Health, 15.07.2019 04:30


 that must be dissolved is 6.89 grams.
that must be dissolved is 6.89 grams.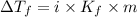
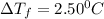 = Depression in freezing point
= Depression in freezing point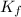 = freezing point constant =
= freezing point constant =