
Chemistry, 22.09.2019 05:30 rosehayden21
The n2o4−no2 reversible reaction is found to have the following equilibrium partial pressures at 100∘c. calculate kp for the reaction. n2o4(g)⇌2no2(g) 0.0005 atm 0.095 atm express your answer using two significant figures.

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2

Chemistry, 23.06.2019 01:10
Can someone check my work 98 5.05 acids and bases for this assignment you will be comparing acids and bases. the chart below will you organize the information needed: acids bases chemical properties (2) deodorant detergent vinger dish soap physical properties (2) orange juice toilet cleaner drain cleaner window cleaner ph level acid ph goes from 0-4 bases ph goes from 10-14 examples around you (2) vinger coffee lemon juice dark chocolate
Answers: 3
You know the right answer?
The n2o4−no2 reversible reaction is found to have the following equilibrium partial pressures at 100...
Questions

Social Studies, 03.10.2019 10:00

Social Studies, 03.10.2019 10:00

Health, 03.10.2019 10:00


Mathematics, 03.10.2019 10:00




Chemistry, 03.10.2019 10:00





Mathematics, 03.10.2019 10:00



English, 03.10.2019 10:00

History, 03.10.2019 10:00

Mathematics, 03.10.2019 10:00

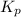 for the reaction is 18.05
for the reaction is 18.05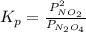
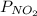 and
and  are equilibrium partial pressure of
are equilibrium partial pressure of 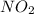 and
and 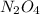 respectively
respectively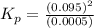 = 18.05
= 18.05

