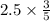Chemistry, 22.09.2019 04:30 sanociahnoel
The combustion of propane (c3h8) in the presence of excess oxygen yields co2 and h20: c3h8 (8) + 5o2 (g) - 3co2 (s) + 4h20 (8) when 2.5 mol of o2 are consumed in their reaction, a) 3.0 b) 7.5 c) 1.5 mol of co2 are produced. d) 4.2 e) 2.5

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
If a bottle of olive oil contains 1.4 kg of olive oil, what is the volume, in milliliters ( ml ), of the olive oil?
Answers: 1

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 04:50
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 22.06.2019 06:00
Calculate the mass of silver needed to react with chlorine to produce 126g if silver chloride?
Answers: 3
You know the right answer?
The combustion of propane (c3h8) in the presence of excess oxygen yields co2 and h20: c3h8 (8) + 5o...
Questions







Mathematics, 20.02.2020 23:29












Mathematics, 20.02.2020 23:29