

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 17:30
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1

Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1

Chemistry, 23.06.2019 09:30
What is the best describtion of the side of the moon that faces earth?
Answers: 1
You know the right answer?
Calculate the enthalpy change for the reaction: caf2 + h2so4 → 2hf + caso4 given that enthalpy chan...
Questions

Mathematics, 17.12.2020 19:00

Mathematics, 17.12.2020 19:00


Mathematics, 17.12.2020 19:00

Mathematics, 17.12.2020 19:00

Mathematics, 17.12.2020 19:00

History, 17.12.2020 19:00



Mathematics, 17.12.2020 19:00

English, 17.12.2020 19:00

Mathematics, 17.12.2020 19:00

Spanish, 17.12.2020 19:00

Mathematics, 17.12.2020 19:00



Chemistry, 17.12.2020 19:00

English, 17.12.2020 19:00

Mathematics, 17.12.2020 19:00

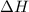
![\Delta H_{rxn}=\sum [n\times \Delta H_f(product)]-\sum [n\times \Delta H_f(reactant)]](/tpl/images/0251/0819/db29b.png)
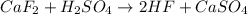
![\Delta H_{rxn}=[(2\times \Delta H_f_{(HF)})+(1\times \Delta H_f_{(CaSO_4)})]-[(1\times \Delta H_f_{(CaF_2)})+(1\times \Delta H_f_{(H_2SO_4)})]](/tpl/images/0251/0819/885d7.png)
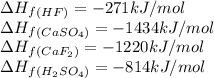
![\Delta H_{rxn}=[(2\times (-271))+(1\times (-1434))]-[(1\times (-1220))+(1\times (-814))]\\\\\Delta H_{rxn}=58kJ](/tpl/images/0251/0819/3f566.png)


