
Chemistry, 22.09.2019 03:20 shealynh52
Considering the limiting reactant, what is the mass of iron produced from 80.0 g of iron(ii)oxide (71.55 g/mol) and 20.0 g of magnesium metal? feof)+ mg() fe)mgo6) a) 62

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:00
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2

Chemistry, 22.06.2019 12:00
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1

Chemistry, 22.06.2019 21:30
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3
You know the right answer?
Considering the limiting reactant, what is the mass of iron produced from 80.0 g of iron(ii)oxide (7...
Questions



Biology, 22.05.2020 00:04





Mathematics, 22.05.2020 00:04

Health, 22.05.2020 00:04

Mathematics, 22.05.2020 00:04

Mathematics, 22.05.2020 00:04


Physics, 22.05.2020 00:04

Mathematics, 22.05.2020 00:04


History, 22.05.2020 00:04




Mathematics, 22.05.2020 00:04

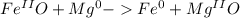
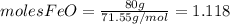 and
and 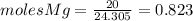 the molecular weight of Mg (24.305) can be readed in the periodic table of elements.so we divide the moles by stoichiometry number (number in front of each compound in the equation) in this case is 1 for both reactants (that is we need 1 mol of FeO and 1 mol of Mg to produce 1 mol of Fe).The lower number obtained was 0.823 for Mg, so Mg is the limiting reactant.
the molecular weight of Mg (24.305) can be readed in the periodic table of elements.so we divide the moles by stoichiometry number (number in front of each compound in the equation) in this case is 1 for both reactants (that is we need 1 mol of FeO and 1 mol of Mg to produce 1 mol of Fe).The lower number obtained was 0.823 for Mg, so Mg is the limiting reactant.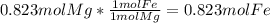 ). To convert from mol of Fe to grams of Fe we would multiply by the molecular weight of Fe
). To convert from mol of Fe to grams of Fe we would multiply by the molecular weight of Fe 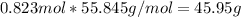 (molecular weight of Fe is readed in the periodic table of elements). So it is produced 45.95 g of iron
(molecular weight of Fe is readed in the periodic table of elements). So it is produced 45.95 g of iron

