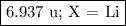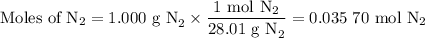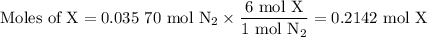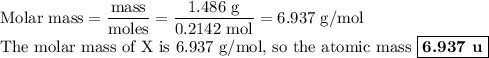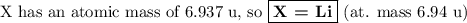Chemistry, 21.09.2019 07:30 MadiAbbott798
Nitrogen reacts with a metal to form a compound in which there are three atoms of the metal for each atom of nitrogen. if 1.486
g of the metal reacts with 1.000 g of nitrogen, what is the calculated atomic mass of the metal?
use your calculated atomic mass to identify the metal. (for your answer, input the proper chemical symbol for element x.)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3

Chemistry, 22.06.2019 19:30
Describe the forces both attractive and repulsive that occur as two atoms move closer together.
Answers: 1

Chemistry, 22.06.2019 21:30
Plzz a sample of table sugar (sucrose, c12h22o11) has a mass of 7.801 g. ● a) calculate the number of moles of c12h22o11 in the sample b) calculate the number of moles of each element in c12h22o11 (number of moles of c, number of moles of h & number of moles of o) in the sample. (use your answer from part a as your starting point.) show your work and highlight your final answer. calculate the number of atoms of each element in c12h22o11 (number of atoms of c, number of atoms of h & number of atoms of o) in the sample. (use your answers from part b as your starting for each element.) show your work and highlight your final answer.
Answers: 1

Chemistry, 23.06.2019 01:00
Heat energy, carbon dioxide, and water are released through which process? a. photosynthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1
You know the right answer?
Nitrogen reacts with a metal to form a compound in which there are three atoms of the metal for each...
Questions


Mathematics, 04.07.2019 20:30

Mathematics, 04.07.2019 20:30





Mathematics, 04.07.2019 20:30

Mathematics, 04.07.2019 20:30

Mathematics, 04.07.2019 20:30

Mathematics, 04.07.2019 20:30

Mathematics, 04.07.2019 20:30

Mathematics, 04.07.2019 20:30

Mathematics, 04.07.2019 20:30

Mathematics, 04.07.2019 20:30

English, 04.07.2019 20:30

Chemistry, 04.07.2019 20:30


Mathematics, 04.07.2019 20:30