
Chemistry, 20.09.2019 17:30 tmontefalcon2424
Bleach contains the active ingredient naclo. analysis of bleach involves two sequential redox reactions: first, bleach is reacted in acidic solution with excess iodide anion to produce yellow-colored iodine: clo− + 2 h+ + 2 i− → i2 + cl− + h2o then, to determine how much of the iodine was formed, the solution is titrated with sodium thiosulfate solution: i2 + 2 s2o32− → 2 i− + s4o62− a bit of starch is added to the titration reaction. starch is intensely blue in the presence of i2. the solution thus turns from deep blue to colorless at the reaction equivalence point. a sample of a new cleaning product, "joe's famous bleach cleaner," with a mass of 60 g , was diluted with an acidic solution containing excess i−. a small amount of starch indicator solution was then added, turning the solution a deep bluish-purple. the solution was then titrated with 0.117 m sodium thiosulfate, na2s2o3, containing the ion s2o32−. a volume of 43 ml of sodium thiosulfate, the titrant, was needed to turn the solution colorless. what is the percentage composition by mass of naclo in the bleach product?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:40
If the atomic mass of an atom is 34 and the atom contains 13 protons, how many neutrons does the atom contain?
Answers: 2

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2

Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1

Chemistry, 22.06.2019 22:30
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1. part a part complete what is the half-life for this reaction?
Answers: 3
You know the right answer?
Bleach contains the active ingredient naclo. analysis of bleach involves two sequential redox reacti...
Questions

Computers and Technology, 16.11.2020 04:30




Mathematics, 16.11.2020 04:30



History, 16.11.2020 04:30

Mathematics, 16.11.2020 04:30



History, 16.11.2020 04:30

Chemistry, 16.11.2020 04:30






Social Studies, 16.11.2020 04:30

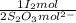 = 2,5156x10⁻³ moles of I₂
= 2,5156x10⁻³ moles of I₂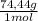 = 0,187 g of NaClO
= 0,187 g of NaClO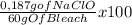 = 0,31%
= 0,31%

