
Chemistry, 20.09.2019 17:30 student0724
Akcl solution is prepared by dissolving 40.0 g kcl (molar mass = 74.55 g/mol) in 250.0 g of water (molar mass = 18.01 g/mol) at 25°c. what is the vapor pressure of the solution if the vapor pressure of water at 25°c is 23.76 mm hg?
a) 20.5 mm hg
b) 22.1 mm hg
c) 22.9 mm hg
d) 24.7 mm hg

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 11:00
Which statement is true about hcl? (5 points) select one: a. it is a salt because it increases the concentration of metallic ions. b. it is a salt because it is formed by the reaction of an acid and a base. c. it is an acid because it increases the concentration of hydroxyl ions. d. it is an acid because it increases the concentration of hydronium ions.
Answers: 1

Chemistry, 22.06.2019 11:30
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2

Chemistry, 22.06.2019 14:30
Ahypothesis must be testable and falsifiable to be considered scientific a. trueb. false
Answers: 1
You know the right answer?
Akcl solution is prepared by dissolving 40.0 g kcl (molar mass = 74.55 g/mol) in 250.0 g of water (m...
Questions

Mathematics, 21.07.2019 09:00




Biology, 21.07.2019 09:00

Mathematics, 21.07.2019 09:00



English, 21.07.2019 09:00


Computers and Technology, 21.07.2019 09:00

Chemistry, 21.07.2019 09:00




History, 21.07.2019 09:00




Mathematics, 21.07.2019 09:00

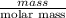
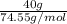
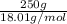
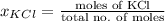
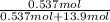
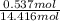
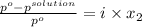
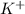 and
and 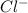 .
.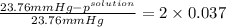
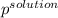 = 22.1 mm Hg
= 22.1 mm Hg

