Chemistry, 20.09.2019 04:00 bryantpropst1395
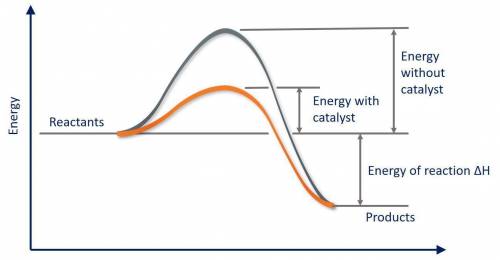
Which of the following statements about catalysts is false? a catalyst provides a different reaction pathway with a lower activation energy. a catalyst cannot affect the overall energy change for the reaction. a catalyst speeds up both the forward and reverse reactions. a catalyst is present before the reaction begins, and is also present in the same form after the reaction ends. a catalyst stabilizes the product of the reaction relative to the reactant.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Based on the equation and the information in the table, what is the enthalpy of the reaction?
Answers: 2

Chemistry, 22.06.2019 03:30
Select the correct answer. when carbon dioxide dissolves in water, it sometimes reacts with water to form carbonic acid as in this balanced equation: co2 + h2o → h2co3. if 495 milliliters of carbon dioxide at 25°c and 101.3 kilopascals reacts with excess water, what is the theoretical yield of carbonic acid? use the periodic table and the ideal gas resource a. 0.889 g b. 1.10g c. 1.27g d. 2.02g what's the answer! quick!
Answers: 1

Chemistry, 22.06.2019 05:50
What happens when the temperature of a solution increases?
Answers: 2

Chemistry, 23.06.2019 00:00
What is the approximate mass of 25 cm3 of silver, if the density is 10.5 g/cm3? a. 0.42 g b. 2.4 g c. 42 g d. 260 g
Answers: 1
You know the right answer?
Which of the following statements about catalysts is false? a catalyst provides a different reactio...
Questions

Mathematics, 05.05.2021 19:00

Mathematics, 05.05.2021 19:00


Mathematics, 05.05.2021 19:00

Mathematics, 05.05.2021 19:00


Chemistry, 05.05.2021 19:00

Mathematics, 05.05.2021 19:00

Mathematics, 05.05.2021 19:00


Physics, 05.05.2021 19:00

History, 05.05.2021 19:00

Arts, 05.05.2021 19:00


Social Studies, 05.05.2021 19:00

Mathematics, 05.05.2021 19:00




Biology, 05.05.2021 19:00