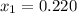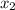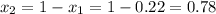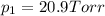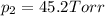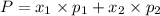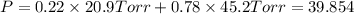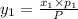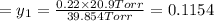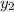Chemistry, 20.09.2019 03:00 aljalloh94
1‑propanol (p∘1=20.9 torr at 25 ∘c) and 2‑propanol (p∘2=45.2 torr at 25 ∘c) form ideal solutions in all proportions. let x1 and x2 represent the mole fractions of 1‑propanol and 2‑propanol in a liquid mixture, respectively, and y1 and y2 represent the mole fractions of each in the vapor phase. for a solution of these liquids with x1=0.220, calculate the composition of the vapor phase at 25 ∘c.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 12:30
Which element has the lowest electronegativity? calcium(ca) gallium(ga) selenium(se) bromine(br)
Answers: 1

Chemistry, 22.06.2019 21:30
Athe top of a hill, an athlete on a skateboard has x joules of mechanical energy. how much mechanical energy will she have at the bottom of the hill? ignore the effects of friction.
Answers: 1

You know the right answer?
1‑propanol (p∘1=20.9 torr at 25 ∘c) and 2‑propanol (p∘2=45.2 torr at 25 ∘c) form ideal solutions in...
Questions

Mathematics, 13.09.2020 15:01

Mathematics, 13.09.2020 16:01

English, 13.09.2020 16:01

Geography, 13.09.2020 16:01

Mathematics, 13.09.2020 16:01

Mathematics, 13.09.2020 16:01

Mathematics, 13.09.2020 16:01

Mathematics, 13.09.2020 16:01

Mathematics, 13.09.2020 16:01

Mathematics, 13.09.2020 16:01

Physics, 13.09.2020 16:01

English, 13.09.2020 16:01

English, 13.09.2020 16:01

Mathematics, 13.09.2020 16:01

Mathematics, 13.09.2020 16:01

Mathematics, 13.09.2020 16:01

Mathematics, 13.09.2020 16:01

Mathematics, 13.09.2020 16:01

Mathematics, 13.09.2020 16:01

Mathematics, 13.09.2020 16:01