
Chemistry, 20.09.2019 02:00 bammbamm538
Write the equilibrium constant expression for the following reaction in terms of concentrations of the components. (concentration equilibrium expressions take the general form: kc = [c]c / [a]a . [b]b. subscripts and superscripts that include letters must be enclosed in braces {}.) mgco3(s) equilibrium reaction arrow mgo(s) + co2(g)

Answers: 1


Another question on Chemistry



Chemistry, 23.06.2019 00:00
How is the way a mixture is combined different from how a compound is combined?
Answers: 3

Chemistry, 23.06.2019 07:20
Which of the following are acids or bases? 1. sodium hydrogen 2. barium hydroxide solution 3. carbonate solution
Answers: 1
You know the right answer?
Write the equilibrium constant expression for the following reaction in terms of concentrations of t...
Questions

Mathematics, 14.11.2020 04:00


Mathematics, 14.11.2020 04:00

Mathematics, 14.11.2020 04:00

Mathematics, 14.11.2020 04:00

Chemistry, 14.11.2020 04:00



Arts, 14.11.2020 04:00


Arts, 14.11.2020 04:00


Social Studies, 14.11.2020 04:00

Advanced Placement (AP), 14.11.2020 04:00


Mathematics, 14.11.2020 04:00


Social Studies, 14.11.2020 04:00

English, 14.11.2020 04:00

Arts, 14.11.2020 04:00

![K_c=[CO_2]](/tpl/images/0244/8923/0ff0c.png)
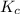
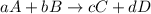
![K_{c}=\frac{[C]^c[D]^d}{[A]^a[B]^b}](/tpl/images/0244/8923/b6f47.png)
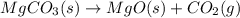
![K_{c}=\frac{[MgO][CO_2]}{[MgCO_3]}](/tpl/images/0244/8923/c8e23.png)
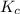 becomes:
becomes:![K_{c}=[CO_2]](/tpl/images/0244/8923/0a298.png)


