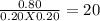Chemistry, 20.09.2019 00:00 corrineikerd
Two different proteins x and y are dissolved in aqueous solution at 37 °c. the proteins bind in a 1: 1 ratio to form xy. a solution that is initially 1.00 mm in each protein is allowed to reach equilibrium. at equilibrium, 0.20 mm of free x and 0.20 mm of free y remain. what is kc for the reaction?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which element forms an ionic bond with flourine? 1) fluorine 2) carbon 3) potassium 4) oxygen
Answers: 1

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2


Chemistry, 23.06.2019 06:30
Consider the heating curve of h2o and line segments a, b, and c. several changes are taking place at a, b, and c. all but one would be an appropriate description as e move through segments a, b and then c.
Answers: 3
You know the right answer?
Two different proteins x and y are dissolved in aqueous solution at 37 °c. the proteins bind in a 1:...
Questions


Mathematics, 27.08.2020 22:01


Law, 27.08.2020 22:01

Mathematics, 27.08.2020 22:01


Social Studies, 27.08.2020 22:01



Biology, 27.08.2020 22:01

History, 27.08.2020 22:01


Mathematics, 27.08.2020 22:01

History, 27.08.2020 22:01



Mathematics, 27.08.2020 22:01

Social Studies, 27.08.2020 22:01

Mathematics, 27.08.2020 22:01

Physics, 27.08.2020 22:01

![\frac{[Products]}{[Reactants]}=\frac{[XY]}{[X][Y]}](/tpl/images/0244/5837/0eb39.png)