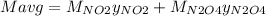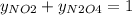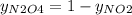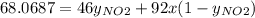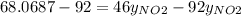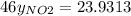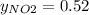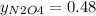Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Asample of the male sex hormone testosterone, c19h28o2, contains 3.88×10^21 atoms of hydrogen.(a) how many atoms of carbon does it contain? (b) how many molecules of testosterone does it contain? (c) how many moles of testosterone does it contain? (d) what is the mass of this sample in grams?
Answers: 1


Chemistry, 23.06.2019 00:30
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1

You know the right answer?
Nitrogen dioxide (no2) cannot be obtained in a pure form in the gas phase because it exists as a mix...
Questions

Mathematics, 19.04.2020 04:25

Mathematics, 19.04.2020 04:25

English, 19.04.2020 04:25

Mathematics, 19.04.2020 04:25


Mathematics, 19.04.2020 04:25


English, 19.04.2020 04:25

Geography, 19.04.2020 04:25



Advanced Placement (AP), 19.04.2020 04:25

Social Studies, 19.04.2020 04:25

Mathematics, 19.04.2020 04:25


Mathematics, 19.04.2020 04:25




Mathematics, 19.04.2020 04:26

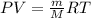
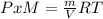
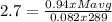
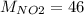 g/mol and
g/mol and 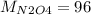 g/mol. Calling y the molar fraction:
g/mol. Calling y the molar fraction: