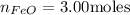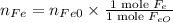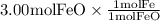Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Agas occupies 475 cm^3 at 313k. find its volume at 367k. you must show all of your work to receive credit. be sure to identify which of the gas laws you will be using
Answers: 2

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3

Chemistry, 22.06.2019 19:20
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3

Chemistry, 22.06.2019 22:30
Write and balance the chemical equation that represents the reaction of aqueous sulfuric acid with aqueous sodium hydroxide to form water and sodium sulfate. include phases.
Answers: 1
You know the right answer?
The reaction between iron(ii) oxide and carbon monoxide produces iron and carbon dioxide. how many m...
Questions

Mathematics, 06.01.2021 17:50




Mathematics, 06.01.2021 17:50

Mathematics, 06.01.2021 17:50

Mathematics, 06.01.2021 17:50

Chemistry, 06.01.2021 17:50

Mathematics, 06.01.2021 17:50

Mathematics, 06.01.2021 17:50

Mathematics, 06.01.2021 17:50


Social Studies, 06.01.2021 17:50

Mathematics, 06.01.2021 17:50


English, 06.01.2021 17:50


Mathematics, 06.01.2021 17:50

Advanced Placement (AP), 06.01.2021 17:50

Mathematics, 06.01.2021 17:50