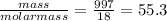Liquids and solids are left out of the equilibrium constant expression because their concentrations remain constant during reactions. what is the molarity concentration of liquid water at 25.0 latex: ^\circ ∘c given that its density is 0.997 g/ml at that temperature?
a.23.5 m
b.0.997 m
c.0.180 m
d.0.156 m
e.55.3 m

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
What layer of the atmosphere is directly above the troposphere?
Answers: 1

Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3

Chemistry, 22.06.2019 23:30
What are the similarities between compounds and mixtures?
Answers: 3

Chemistry, 23.06.2019 00:30
The molecular weight of carbon dioxide, co2, is 44.00 amu, and the molecular weight of nitrous dioxide, no2, is 46.01 amu, so no2 diffuses co2
Answers: 2
You know the right answer?
Liquids and solids are left out of the equilibrium constant expression because their concentrations...
Questions





Mathematics, 09.01.2022 14:00

Mathematics, 09.01.2022 14:00

Biology, 09.01.2022 14:00

Biology, 09.01.2022 14:00


English, 09.01.2022 14:00


History, 09.01.2022 14:00

Biology, 09.01.2022 14:00



Mathematics, 09.01.2022 14:00

Social Studies, 09.01.2022 14:00



Mathematics, 09.01.2022 14:00