
Chemistry, 19.09.2019 20:30 lulabelles7750
Given the following balanced equation at 120°c: a(g) + b(g) ⇋ 2 c(g) + d(s)(a) at equilibrium a 4.0 liter container was found to contain 1.60 moles of a, and 0.40 moles of b, and 0.40 moles of c, and 1.60 moles of d. calculate kc.(b) if 0.20 moles of b and 0.20 mole of c are added to this system, what will be the new equilibrium concentration of a be? (c) if the volume of the container in which the system is at equilibrium [part (a)] is suddenly halved, what will be the new equilibrium concentrations?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:20
Concerning the 10.0 ml of 0.50 m nacl to 100 ml of solution: when a solution is diluted, does it change the number of moles dissolved?
Answers: 3

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3

Chemistry, 22.06.2019 09:00
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2

You know the right answer?
Given the following balanced equation at 120°c: a(g) + b(g) ⇋ 2 c(g) + d(s)(a) at equilibrium a 4.0...
Questions


Mathematics, 16.12.2020 02:50



English, 16.12.2020 02:50

Biology, 16.12.2020 02:50

Health, 16.12.2020 02:50




Chemistry, 16.12.2020 02:50

Mathematics, 16.12.2020 02:50

Mathematics, 16.12.2020 02:50

History, 16.12.2020 02:50

Mathematics, 16.12.2020 02:50

Social Studies, 16.12.2020 02:50

Mathematics, 16.12.2020 02:50

Mathematics, 16.12.2020 02:50


Engineering, 16.12.2020 02:50

![\frac{[C]^2}{[A][B]}](/tpl/images/0244/0592/0c73c.png)
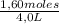 = 0,4 M
= 0,4 M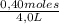 = 0,1 M
= 0,1 M![\frac{[0,1]^2}{[0,4][0,1]}](/tpl/images/0244/0592/6198c.png) = 0,25
= 0,25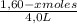
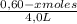
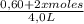
![\frac{[0,60+2x]^2}{[1,60-x][0,60-x]}](/tpl/images/0244/0592/06e92.png)
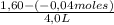 = 0,41 M
= 0,41 M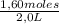 = 0,8 M
= 0,8 M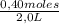 = 0,2 M
= 0,2 M

