
Acompound of formula xcl3 reacts with aqueous agno3 to yield solid agcl according to the following equation: xcl3(aq)+3agno3(aq)→x(no3)3(aq)+3ag cl(s) when a solution containing 0.521 g of xcl3 was allowed to react with an excess of aqueous agno3, 1.68 g of solid agcl was formed. what is the identity of the atom x?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:20
2pos suppose an object in free fall is dropped from a building. its starting velocity is 0 m/s. ignoring the effects of air resistance, what is the speed (in m/s) of the object after falling 3 seconds? give your answer as a positive decimal without units. answer here
Answers: 1

Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2

Chemistry, 22.06.2019 19:00
Suppose that a certain fortunate person has a net worth of $71.0 billion ($7.10×1010). if her stock has a good year and gains $3.20 billion (3.20×109) in value, what is her new net worth?
Answers: 3

Chemistry, 22.06.2019 20:10
What would happen to a volleyball left outside in the winter? o o o o a. it would expand. b. it would lose air. c. it would shrink. d. it would explode.
Answers: 2
You know the right answer?
Acompound of formula xcl3 reacts with aqueous agno3 to yield solid agcl according to the following e...
Questions

Mathematics, 25.08.2021 19:10



Biology, 25.08.2021 19:10

Mathematics, 25.08.2021 19:10



Mathematics, 25.08.2021 19:10

Mathematics, 25.08.2021 19:10



Chemistry, 25.08.2021 19:10

Mathematics, 25.08.2021 19:10

Chemistry, 25.08.2021 19:10

English, 25.08.2021 19:10


Biology, 25.08.2021 19:10

Mathematics, 25.08.2021 19:10


Mathematics, 25.08.2021 19:10

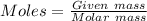
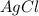 = 1.68 g
= 1.68 g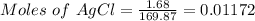
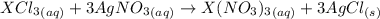
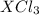 undergoes reaction.
undergoes reaction.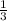 mole of
mole of 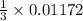 mole of
mole of 


