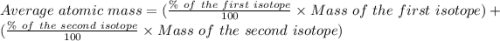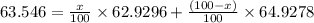Chemistry, 19.09.2019 16:30 xxaurorabluexx
Copper has two naturally occurring isotopes, 63cu (isotopic mass 62.9296 amu) and 65cu (isotopic mass 64.9278 amu). if copper has an atomic mass of 63.546 amu, what is the percent abundance of each isotope? report your answer to 5 significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:50
The electron configuration for chromium is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 5 4 s 1 instead of 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 4 4 s 1 . the configuration is an exception to the
Answers: 3

Chemistry, 21.06.2019 21:30
If i make a solution by adding 83grams of sodium hydroxide to 750ml i’d water what is the molarity of sodium hydroxide
Answers: 1

Chemistry, 22.06.2019 03:40
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3

Chemistry, 22.06.2019 14:20
Which of the following are sources of revenue for media companies? a. direct sales to producers b.advertising and subscriptions c. online purchase d. capital investments
Answers: 1
You know the right answer?
Copper has two naturally occurring isotopes, 63cu (isotopic mass 62.9296 amu) and 65cu (isotopic mas...
Questions

Social Studies, 26.10.2021 04:20

Chemistry, 26.10.2021 04:20



Mathematics, 26.10.2021 04:20



Mathematics, 26.10.2021 04:20


Mathematics, 26.10.2021 04:20

Medicine, 26.10.2021 04:20

Geography, 26.10.2021 04:20







Health, 26.10.2021 04:20

Mathematics, 26.10.2021 04:20