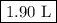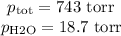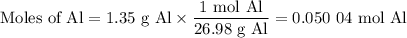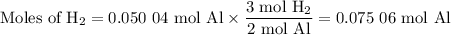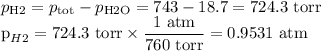Chemistry, 19.09.2019 03:00 davelopez979
Suppose we now collect hydrogen gas, h2(g), over water at 21◦c in a vessel with total pressure of 743 torr. if the hydrogen gas is produced by the reaction of aluminum with hydrochloric acid:
2al(s) + 6hcl(aq) → 2alcl3(aq) + 3h2(g)
what volume of hydrogen gas will be collected if 1.35 g al(s) reacts with excess hcl(aq)? express
your answer in liters.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2

Chemistry, 22.06.2019 06:30
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2
You know the right answer?
Suppose we now collect hydrogen gas, h2(g), over water at 21◦c in a vessel with total pressure of 74...
Questions


Physics, 16.11.2020 23:10

Biology, 16.11.2020 23:10


History, 16.11.2020 23:10

Mathematics, 16.11.2020 23:10

Mathematics, 16.11.2020 23:10


Social Studies, 16.11.2020 23:10

Arts, 16.11.2020 23:10


English, 16.11.2020 23:10


History, 16.11.2020 23:10


Mathematics, 16.11.2020 23:10


Mathematics, 16.11.2020 23:10

Mathematics, 16.11.2020 23:10