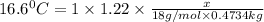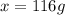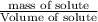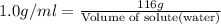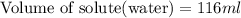Chemistry, 18.09.2019 22:00 shoafmckenzie5263
What volume of water (solute) (d = 1.00 g/ml) should be added to 600. ml of ethanol (solvent, c2h5oh) in order to have a solution that boils at 95.0°c? [for ethanol, kb = 1.22 °c/m, density = 0.789 g/ ml, boiling point = 78.4°c]

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:00
The density of a planet is 0.69 g/cm3 (density of water is 1.0 g/cm3). which of the following planets might this be? a. mercury b. venus c. saturn d. mars
Answers: 3

Chemistry, 22.06.2019 00:00
How many liters of water vapor can be produced if 108 grams of methane gas (ch4) are combusted at 312 k and 0.98 atm? show all work. pls ! will mark as brainliest
Answers: 1

Chemistry, 22.06.2019 01:30
Reaction rate depends on how many molecules are coming into contact with each other with enough energy to react. increasing the temperature of the reactants will increase -
Answers: 3

You know the right answer?
What volume of water (solute) (d = 1.00 g/ml) should be added to 600. ml of ethanol (solvent, c2h5oh...
Questions


Mathematics, 19.08.2019 02:20


English, 19.08.2019 02:20

Mathematics, 19.08.2019 02:20


English, 19.08.2019 02:20

Mathematics, 19.08.2019 02:20

Biology, 19.08.2019 02:20









Mathematics, 19.08.2019 02:20


Mathematics, 19.08.2019 02:20

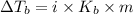
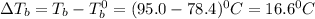 = elevation in boiling point
= elevation in boiling point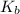 =boiling point constant =
=boiling point constant = 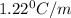
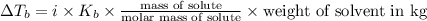
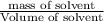
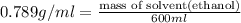
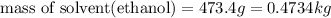 (1kg=1000g)
(1kg=1000g)