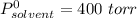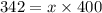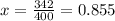Chemistry, 18.09.2019 17:20 lindseyreneesmith7
Diethyl ether has a vapor pressure of 400.0 torr at 18°c. when a sample of benzoic acid (a non-volatile compound) is dissolved in ether, the vapor pressure of the solution is 342 torr. what is the mole fraction of benzoic acid in the solution?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:20
Both 1,2−dihydronaphthalene and 1,4−dihydronaphthalene may be selectively hydrogenated to 1,2,3,4−tetrahydronaphthalene. one of these isomers has a heat of hydrogenation of 101 kj/mol (24.1 kcal/mol), and the heat of hydrogenation of the other is 113 kj/mol (27.1 kcal/mol). match the heat of hydrogenation with the appropriate dihydronaphthalene.
Answers: 2

Chemistry, 22.06.2019 07:00
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2


Chemistry, 22.06.2019 18:00
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3
You know the right answer?
Diethyl ether has a vapor pressure of 400.0 torr at 18°c. when a sample of benzoic acid (a non-volat...
Questions

Chemistry, 20.06.2020 15:57

Biology, 20.06.2020 16:57

Biology, 20.06.2020 16:57









Computers and Technology, 20.06.2020 16:57

History, 20.06.2020 16:57

English, 20.06.2020 16:57





Physics, 20.06.2020 16:57

Business, 20.06.2020 16:57